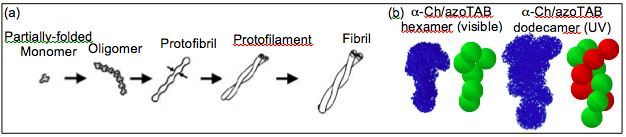
A variety of neurodegenerative diseases, including Alzheimer’s, Huntington’s, Parkinson’s, and prion diseases, are associated with amyloid fibrillogenesis where proteins self-associate into insoluble fibrils. As shown below, fibril formation begins with an unstable, partially-folded monomer that results in a cascading aggregation process leading to oligomers (often hexamers), short protofibrils (10 – 200 nm long),protofilaments (two intertwined protofibrils), and eventually fibrils (2 – 6 intertwined protofilaments). In Alzheimer’s, for example, extracellular senile plaques are composed of aggregated fibrils that form in the diseased brain. As a result, early work in this field focused on characterization of the fibril structure as well the prevention of fully-developed fibrils. However, recent studies have shown that synaptic activity is primarily affected by soluble intermediates well before amyloid aggregation into fibril plaques. Thus, oligomers and protofibrils have become increasingly viewed as the primary pathogenic species.
Unfortunately, the in vitro structure of these important intermediates has, until recently, remained unknown. X-ray crystallography and solution NMR are limited to native proteins or relatively small protein assemblies (<30 kDa), respectively. Conversely, AFM and TEM are better suited for larger (protofilament or fibril) structures that are less sensitive to drying or surface effects. Thus, a need exists for novel methods capable of characterizing protein oligomers and protofibrils in this intermediate size range.
To investigate the conformations of amyloid intermediates we have developed two complementary approaches: (1) a means to induce changes in protein folding and, hence, association in a controlled and reversible manner with light illumination, and (2) an approach based on small-angle neutron scattering (SANS) to determine the in vitroconformation of non-native and associated proteins. These methods utilize the interaction of proteins with photosensitive surfactants that can be switched “on” or “off” with light. Thus, light can be used to reversibly bind surfactants to proteins, leading to photocontrol of protein conformation. This is illustrated below for a-chymotrypsin, found to form corkscrew-like hexamers under visible light (surfactant switched “on”) and rope-like dodecamers of two intertwined hexamers under UV light (surfactant switched “off”). Not surprisingly, incubation leads to twisted amyloid fibrils of the type shown in the figure below.
Selected Publications:
1. Hamill, A.C.; Lee, C.T. “Photocontrol of β-Amyloid Peptide (1-40) Fibril Growth in the Presence of a Photosurfactant” J. Phys. Chem. B 2009, 113, 6164
2. Hamill, A.C.; Lee, C.T. “Solution Structure of an Amyloid-Forming Protein During Photoinitiated Hexamer-Dodecamer Transitions Revealed through Small-Angle Neutron Scattering” Biochemistry 2007, 46, 7694